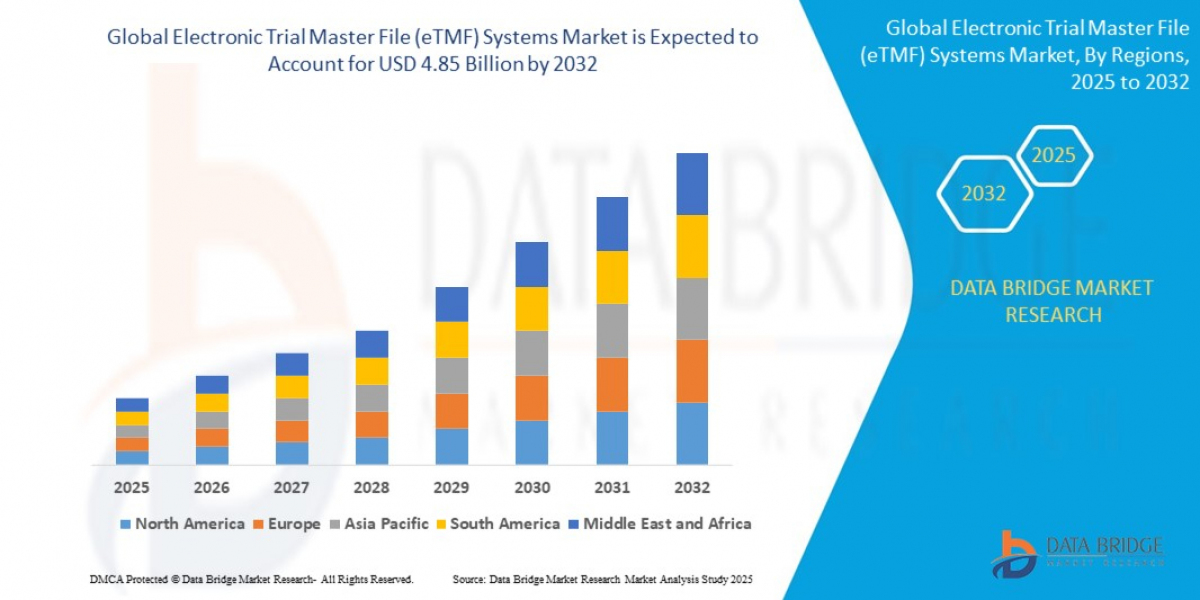
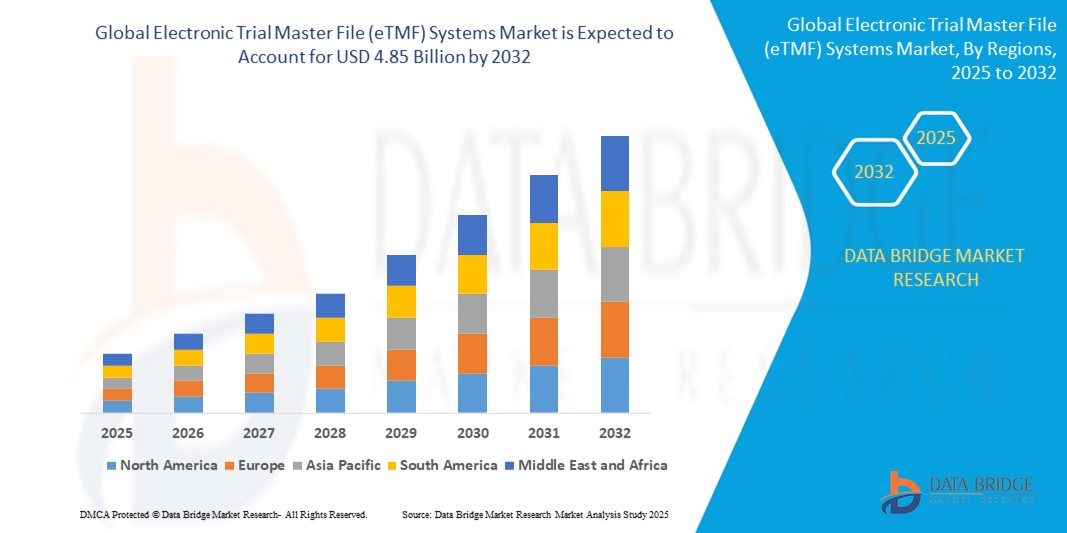
"Executive Summary Electronic Trial Master File (eTMF) Systems Market Trends: Share, Size, and Future Forecast
- The global electronic trial master file (eTMF) systems market size was valued at USD 1.84 billion in 2024 and is expected to reach USD 4.85 billion by 2032, at a CAGR of 12.90% during the forecast period
 By working with a number of steps of collecting and analysing market data, the significant Electronic Trial Master File (eTMF) Systems Market research report is framed with the expert team. Being an outstanding resource of market info, the report provides recent as well as upcoming technical and financial details of the industry. The market study and analysis of this business report also lends a hand to figure out types of consumers, their views about the product, their buying intentions and their ideas for advancement of a product. The world class Electronic Trial Master File (eTMF) Systems Market report comprises of various segments linked to Electronic Trial Master File (eTMF) Systems Market industry and market with comprehensive research and analysis.
By working with a number of steps of collecting and analysing market data, the significant Electronic Trial Master File (eTMF) Systems Market research report is framed with the expert team. Being an outstanding resource of market info, the report provides recent as well as upcoming technical and financial details of the industry. The market study and analysis of this business report also lends a hand to figure out types of consumers, their views about the product, their buying intentions and their ideas for advancement of a product. The world class Electronic Trial Master File (eTMF) Systems Market report comprises of various segments linked to Electronic Trial Master File (eTMF) Systems Market industry and market with comprehensive research and analysis.
An all-inclusive Electronic Trial Master File (eTMF) Systems Market research report directs the manufacturer about planning of advertising and sales promotion efforts and makes it more effective. The report consists of all the detailed profiles for the Electronic Trial Master File (eTMF) Systems Market’s major manufacturers and importers who are influencing the market. This market survey report provides key information about the Electronic Trial Master File (eTMF) Systems Market industry such as helpful and important facts and figures, expert opinions, and the latest developments across the globe. An influential Electronic Trial Master File (eTMF) Systems Market study includes drivers and restraints for the market along with the impact they have on the demand over the forecast period derived with the help of SWOT analysis.
Examine detailed statistics, forecasts, and expert analysis in our Electronic Trial Master File (eTMF) Systems Market report. Download now:
https://www.databridgemarketresearch.com/reports/global-electronic-trial-master-file-etmf-systems-market
Electronic Trial Master File (eTMF) Systems Sector Overview
Segments
- Based on component, the global electronic trial master file (eTMF) systems market can be segmented into software and services. The software segment is expected to dominate the market due to the growing adoption of eTMF systems by pharmaceutical companies, CROs, and research organizations to ensure efficient management of clinical trial data. The services segment is also expected to witness significant growth as companies offer implementation, integration, training, and support services to meet the specific needs of clients.
- On the basis of deployment mode, the market can be segmented into cloud-based and on-premise eTMF systems. Cloud-based eTMF systems are gaining traction among stakeholders in the life sciences industry due to benefits such as scalability, flexibility, and cost-effectiveness. On-premise eTMF systems are preferred by organizations that prioritize data security and have specific compliance requirements.
- By end-user, the market can be categorized into pharmaceutical and biotechnology companies, contract research organizations (CROs), and other end users such as medical device companies and academic research institutes. Pharmaceutical and biotechnology companies are the major end users of eTMF systems, as they conduct numerous clinical trials and require efficient management of trial-related documents to comply with regulatory standards.
Market Players
- Some of the key players operating in the global electronic trial master file (eTMF) systems market include Veeva Systems, Oracle Corporation, Phlexglobal Ltd., ArisGlobal LLC, TransPerfect, SureClinical, CareLex, Montrium, MasterControl, Aurea Software, Wingspan Technology, Mayo Clinic, SterlingBio, ePharmaONE, and eTMF Hub. These companies are focusing on strategic partnerships, product enhancements, and geographic expansions to strengthen their market presence and cater to the increasing demand for advanced eTMF solutions.
The global electronic trial master file (eTMF) systems market is experiencing a paradigm shift driven by the increasing focus on digitization and the need for streamlined processes in the life sciences industry. Beyond the traditional segmentation based on components, deployment modes, and end-users, there are emerging trends and opportunities shaping the market landscape. One such trend is the integration of artificial intelligence (AI) and machine learning capabilities into eTMF systems. The use of AI algorithms can automate document management, enhance data quality, and facilitate predictive analytics, ultimately improving operational efficiencies and decision-making processes for stakeholders in clinical trials. Market players are increasingly investing in AI-driven eTMF solutions to stay ahead of the curve and offer advanced functionalities to their clients.
Another significant development in the eTMF systems market is the rising importance of data security and compliance with stringent regulatory requirements. As the volume of clinical trial data continues to grow exponentially, ensuring the confidentiality, integrity, and availability of sensitive information becomes paramount. Market players are innovating with encryption technologies, audit trails, and access controls to safeguard data within eTMF systems. Moreover, with the implementation of regulations like GDPR and HIPAA, companies are under pressure to adhere to data protection standards, creating opportunities for specialized eTMF vendors focusing on compliance solutions.
Furthermore, the market is witnessing a shift towards holistic clinical data management platforms that offer end-to-end solutions beyond traditional eTMF functionalities. Integrated platforms that combine electronic data capture (EDC), clinical trial management systems (CTMS), and eTMF capabilities are gaining traction as they provide seamless data flow, real-time insights, and enhanced collaboration among stakeholders. Market players are increasingly partnering and acquiring companies to create comprehensive clinical data management suites that cater to the evolving needs of the industry.
Moreover, the COVID-19 pandemic has accelerated the adoption of eTMF systems as remote and decentralized trials became the new norm. The need for virtual collaboration, secure data sharing, and remote monitoring capabilities has propelled the demand for cloud-based eTMF solutions that enable seamless access to trial data from anywhere and at any time. As the industry continues to embrace digital transformation, market players are focusing on developing user-friendly interfaces, mobile applications, and data visualization tools to enhance the user experience and drive higher adoption rates.
In conclusion, the global eTMF systems market is poised for significant growth and innovation driven by technological advancements, regulatory complexities, and the evolving needs of the life sciences industry. Market players that can adapt to these dynamics, offer tailored solutions, and provide value-added services will secure a competitive edge in this rapidly expanding market.The global electronic trial master file (eTMF) systems market is witnessing profound transformations driven by technological advancements, evolving regulatory landscapes, and shifting industry dynamics. One of the key trends shaping the market is the integration of artificial intelligence (AI) and machine learning capabilities into eTMF systems. AI algorithms are revolutionizing document management by automating tasks, improving data quality, and enabling predictive analytics, thereby enhancing operational efficiencies and decision-making processes for stakeholders in clinical trials. Market players are increasingly investing in AI-driven eTMF solutions to stay competitive and offer advanced functionalities to meet the growing demand for streamlined processes and enhanced outcomes.
Data security and regulatory compliance are becoming increasingly critical in the eTMF systems market due to the exponential growth of clinical trial data and the need to protect sensitive information. Market players are innovating with encryption technologies, audit trails, and access controls to ensure data integrity and confidentiality within eTMF systems. Furthermore, compliance with regulations like GDPR and HIPAA is pushing companies towards specialized eTMF vendors that provide robust compliance solutions, presenting opportunities for vendors focusing on data protection and regulatory adherence.
A notable shift towards holistic clinical data management platforms is shaping the market, with integrated solutions combining EDC, CTMS, and eTMF functionalities gaining traction. These platforms offer end-to-end solutions that facilitate seamless data flow, real-time insights, and improved collaboration among stakeholders. Market players are actively engaging in partnerships and acquisitions to build comprehensive clinical data management suites that cater to the evolving needs of the industry, indicating a broader market trend towards integrated platforms that offer enhanced functionalities and efficiencies.
The COVID-19 pandemic has accelerated the adoption of eTMF systems, with remote and decentralized trials becoming the new standard. The industry's shift towards virtual collaboration, secure data sharing, and remote monitoring has fueled the demand for cloud-based eTMF solutions that enable seamless access to trial data from anywhere, at any time. Market players are prioritizing the development of user-friendly interfaces, mobile applications, and data visualization tools to enhance the user experience and drive higher adoption rates, aligning with the industry's digital transformation journey.
In conclusion, the global eTMF systems market is poised for substantial growth and innovation, propelled by technological advancements, regulatory complexities, and evolving industry requirements. Market players that can adapt to these trends, offer tailored solutions, and deliver value-added services will position themselves competitively in this dynamic and expanding market landscape.
View company-specific share within the sector
https://www.databridgemarketresearch.com/reports/global-electronic-trial-master-file-etmf-systems-market/companies
Strategic Question Sets for In-Depth Electronic Trial Master File (eTMF) Systems Market Analysis
- What is the reported value of the Electronic Trial Master File (eTMF) Systems Market?
- How is growth in the market expected to evolve annually?
- What submarkets are examined within the broader Electronic Trial Master File (eTMF) Systems Market?
- Who are the major firms setting industry trends?
- What recent advancements are influencing Electronic Trial Master File (eTMF) Systems Market dynamics?
- What nation-specific insights are provided in the Electronic Trial Master File (eTMF) Systems Market report?
- What part of the globe is currently expanding fastest?
- Which country will hold the dominant market role?
- Which market area has the greatest share today?
- Which country is showing record-high CAGR trends?
Browse More Reports:
Global Ready to Drink (RTD) Alcoholic Beverages Market
Global Nanomedicine Market
Global Quinoa Market
West Africa Dairy Market
Global Aesthetic Dermatology Market
Global Luxury Watch Market
Global Metal Roofing Market
Global Smartwatch Market
Global Dairy Market
Global Non Alcoholic Beverages Market
Europe Cosmetics Market
West Africa Baby Food Market
Middle East and Africa Aesthetic Dermatology Market
Global Coffee Machines Market
Global Furniture Fittings Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
"